Calculate the Index of Association and Standardized Index of Association.
Usage
ia(
gid,
sample = 0,
method = 1,
quiet = FALSE,
missing = "ignore",
plot = TRUE,
hist = TRUE,
index = "rbarD",
valuereturn = FALSE
)
pair.ia(
gid,
sample = 0L,
quiet = FALSE,
plot = TRUE,
low = "blue",
high = "red",
limits = NULL,
index = "rbarD",
method = 1L
)
resample.ia(gid, n = NULL, reps = 999, quiet = FALSE, use_psex = FALSE, ...)
jack.ia(gid, n = NULL, reps = 999, quiet = FALSE)Arguments
- gid
a
adegenet::genind()orgenclone()object.- sample
an integer indicating the number of permutations desired (eg 999).
- method
an integer from 1 to 4 indicating the sampling method desired. see
shufflepop()for details.- quiet
Should the function print anything to the screen while it is performing calculations?
TRUEprints nothing.FALSE(default) will print the population name and progress bar.- missing
a character string. see
missingno()for details.- plot
When
TRUE(default), a heatmap of the values per locus pair will be plotted (forpair.ia()). Whensampling > 0, different things happen withia()andpair.ia(). Foria(), a histogram for the data set is plotted. Forpair.ia(), p-values are added as text on the heatmap.- hist
logicalDeprecated. Use plot.- index
charactereither "Ia" or "rbarD". Ifhist = TRUE, this indicates which index you want represented in the plot (default: "rbarD").- valuereturn
logicalifTRUE, the index values from the reshuffled data is returned. IfFALSE(default), the index is returned with associated p-values in a 4 element numeric vector.- low
(for pair.ia) a color to use for low values when `plot = TRUE`
- high
(for pair.ia) a color to use for low values when `plot = TRUE`
- limits
(for pair.ia) the limits to be used for the color scale. Defaults to `NULL`. If you want to use a custom range, supply two numbers between -1 and 1, (e.g. `limits = c(-0.15, 1)`)
- n
an integer specifying the number of samples to be drawn. Defaults to
NULL, which then uses the number of multilocus genotypes.- reps
an integer specifying the number of replicates to perform. Defaults to 999.
- use_psex
a logical. If
TRUE, the samples will be weighted by the value of psex. Defaults toFALSE.- ...
arguments passed on to
psex
Value
for pair.ia()
A matrix with two columns and choose(nLoc(gid), 2) rows representing the values for Ia and rbarD per locus pair.
If no sampling has occurred:
A named number vector of length 2 giving the Index of Association, "Ia"; and the Standardized Index of Association, "rbarD"
If there is sampling:
A a named numeric vector of length 4 with the following values:
Ia - numeric. The index of association.
p.Ia - A number indicating the p-value resulting from a one-sided permutation test based on the number of samples indicated in the original call.
rbarD - numeric. The standardized index of association.
p.rD - A factor indicating the p-value resulting from a one-sided permutation test based on the number of samples indicated in the original call.
Details
ia()calculates the index of association over all loci in the data set.pair.ia()calculates the index of association in a pairwise manner among all loci.resample.ia()calculates the index of association on a reduced data set multiple times to create a distribution, showing the variation of values observed at a given sample size (previouslyjack.ia()).
The index of association was originally developed by A.H.D. Brown analyzing population structure of wild barley (Brown, 1980). It has been widely used as a tool to detect clonal reproduction within populations . Populations whose members are undergoing sexual reproduction, whether it be selfing or out-crossing, will produce gametes via meiosis, and thus have a chance to shuffle alleles in the next generation. Populations whose members are undergoing clonal reproduction, however, generally do so via mitosis. This means that the most likely mechanism for a change in genotype is via mutation. The rate of mutation varies from species to species, but it is rarely sufficiently high to approximate a random shuffling of alleles. The index of association is a calculation based on the ratio of the variance of the raw number of differences between individuals and the sum of those variances over each locus . You can also think of it as the observed variance over the expected variance. If they are the same, then the index is zero after subtracting one (from Maynard-Smith, 1993): $$I_A = \frac{V_O}{V_E}-1$$
Since the distance is more or less a binary distance, any sort of marker can be used for this analysis. In the calculation, phase is not considered, and any difference increases the distance between two individuals. Remember that each column represents a different allele and that each entry in the table represents the fraction of the genotype made up by that allele at that locus. Notice also that the sum of the rows all equal one. Poppr uses this to calculate distances by simply taking the sum of the absolute values of the differences between rows.
The calculation for the distance between two individuals at a single locus with a allelic states and a ploidy of k is as follows (except for Presence/Absence data):
$$ d = \displaystyle \frac{k}{2}\sum_{i=1}^{a} \mid A_{i} - B_{i}\mid$$
To find the total number of differences between two individuals over all loci, you just take d over m loci, a value we'll call D:
$$D = \displaystyle \sum_{i=1}^{m} d_i $$
These values are calculated over all possible combinations of individuals in the data set, \({n \choose 2}\) after which you end up with \({n \choose 2}\cdot{}m\) values of d and \({n \choose 2}\) values of D. Calculating the observed variances is fairly straightforward (modified from Agapow and Burt, 2001):
$$ V_O = \frac{\displaystyle \sum_{i=1}^{n \choose 2} D_{i}^2 - \frac{(\displaystyle\sum_{i=1}^{n \choose 2} D_{i})^2}{{n \choose 2}}}{{n \choose 2}}$$
Calculating the expected variance is the sum of each of the variances of the individual loci. The calculation at a single locus, j is the same as the previous equation, substituting values of D for d:
$$ var_j = \frac{\displaystyle \sum_{i=1}^{n \choose 2} d_{i}^2 - \frac{(\displaystyle\sum_{i=1}^{n \choose 2} d_i)^2}{{n \choose 2}}}{{n \choose 2}} $$
The expected variance is then the sum of all the variances over all m loci:
$$ V_E = \displaystyle \sum_{j=1}^{m} var_j $$
Agapow and Burt showed that \(I_A\) increases steadily with the number of loci, so they came up with an approximation that is widely used, \(\bar r_d\). For the derivation, see the manual for multilocus.
$$ \bar r_d = \frac{V_O - V_E} {2\displaystyle \sum_{j=1}^{m}\displaystyle \sum_{k \neq j}^{m}\sqrt{var_j\cdot{}var_k}} $$
References
Paul-Michael Agapow and Austin Burt. Indices of multilocus linkage disequilibrium. Molecular Ecology Notes, 1(1-2):101-102, 2001
A.H.D. Brown, M.W. Feldman, and E. Nevo. Multilocus structure of natural populations of Hordeum spontaneum. Genetics, 96(2):523-536, 1980.
J M Smith, N H Smith, M O'Rourke, and B G Spratt. How clonal are bacteria? Proceedings of the National Academy of Sciences, 90(10):4384-4388, 1993.
Examples
data(nancycats)
ia(nancycats)
#> Ia rbarD
#> 0.17207262 0.02178965
# Pairwise over all loci:
data(partial_clone)
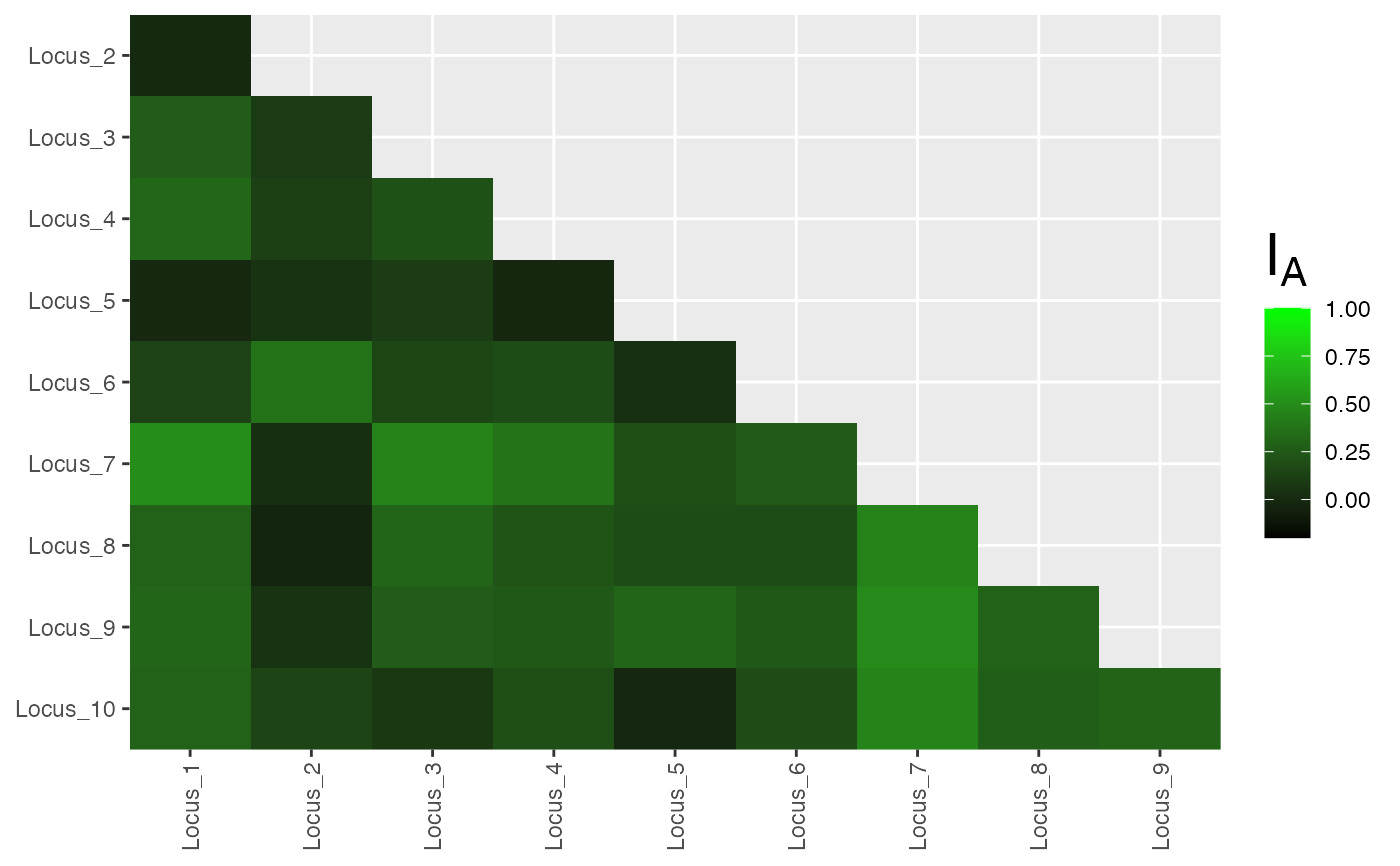
res <- pair.ia(partial_clone)
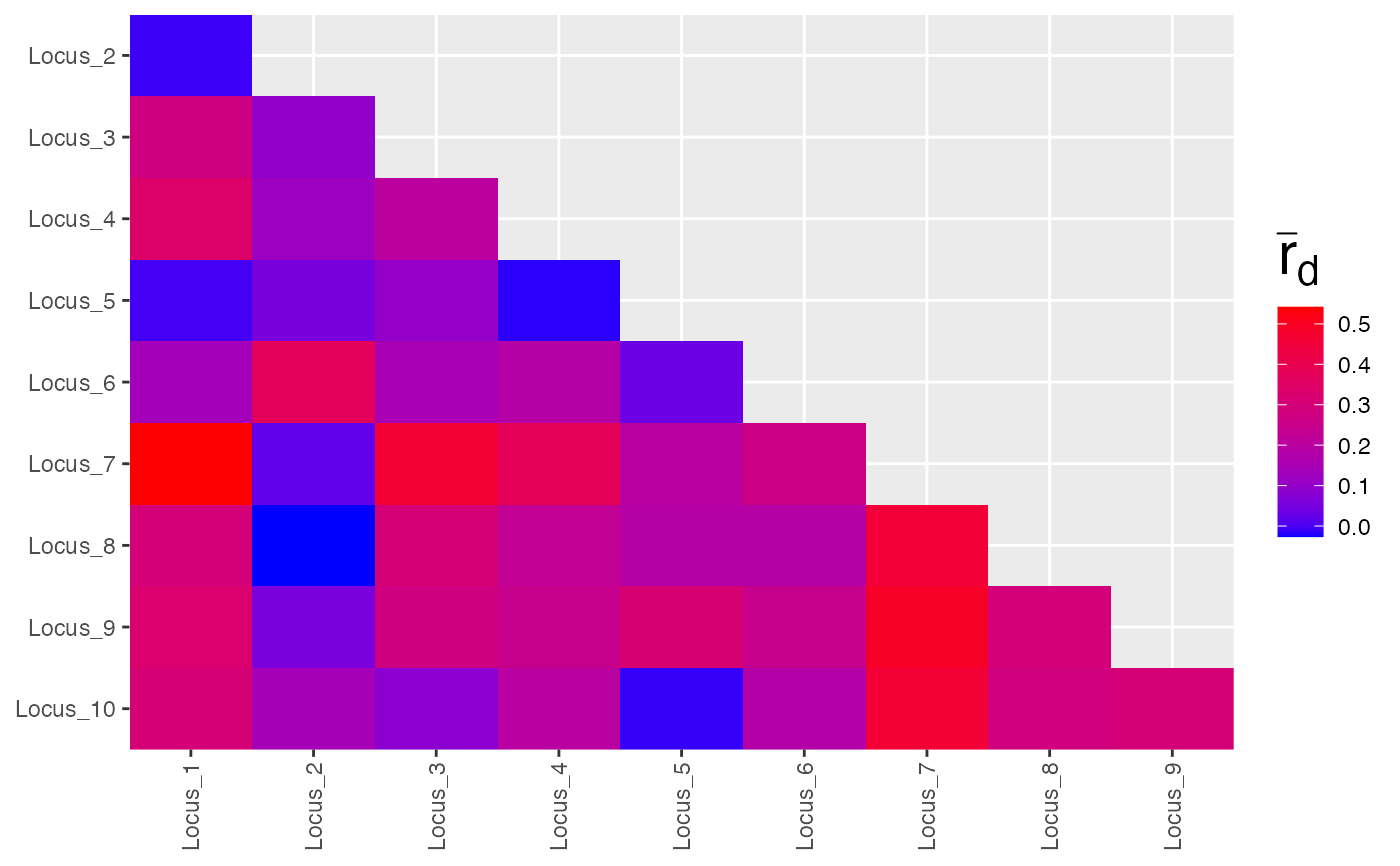 plot(res, low = "black", high = "green", index = "Ia")
plot(res, low = "black", high = "green", index = "Ia")
 # Resampling
data(Pinf)
resample.ia(Pinf, reps = 99)
#> Ia rbarD
#> 1 0.6326707 0.06954708
#> 2 0.6883222 0.07541424
#> 3 0.6740923 0.07387103
#> 4 0.5770239 0.06334574
#> 5 0.6091020 0.06637954
#> 6 0.5727045 0.06257368
#> 7 0.7155065 0.08212542
#> 8 0.6602011 0.07599176
#> 9 0.6471711 0.07096785
#> 10 0.5719934 0.06288824
#> 11 0.7141845 0.07861272
#> 12 0.6605182 0.07202927
#> 13 0.5484642 0.06268892
#> 14 0.7052128 0.07729392
#> 15 0.6276769 0.07197724
#> 16 0.6380409 0.06991244
#> 17 0.6086037 0.06975659
#> 18 0.6020064 0.06584930
#> 19 0.6331831 0.06928114
#> 20 0.6693489 0.07317111
#> 21 0.5524509 0.06033854
#> 22 0.6227598 0.07126893
#> 23 0.6450267 0.07040975
#> 24 0.6631087 0.07652916
#> 25 0.6463076 0.07083580
#> 26 0.6238613 0.06817980
#> 27 0.4787489 0.05220704
#> 28 0.7371110 0.08042796
#> 29 0.5515054 0.06044891
#> 30 0.6232699 0.06820601
#> 31 0.7071895 0.07748315
#> 32 0.6639126 0.07270494
#> 33 0.7081194 0.07737906
#> 34 0.6640895 0.07625090
#> 35 0.5629285 0.06154621
#> 36 0.5949440 0.06501098
#> 37 0.4891759 0.05349182
#> 38 0.6563514 0.07168735
#> 39 0.7800728 0.09000387
#> 40 0.6088116 0.06673665
#> 41 0.5569843 0.06091959
#> 42 0.6360185 0.06954124
#> 43 0.6308747 0.06906819
#> 44 0.7320468 0.08394502
#> 45 0.6802496 0.07450185
#> 46 0.5492261 0.06031984
#> 47 0.6919209 0.07928974
#> 48 0.6616311 0.07240787
#> 49 0.5632249 0.06183149
#> 50 0.6618410 0.07272939
#> 51 0.6293655 0.06914113
#> 52 0.7046288 0.07706778
#> 53 0.6398762 0.07020089
#> 54 0.7105198 0.07794080
#> 55 0.5990801 0.06551082
#> 56 0.7076892 0.07796176
#> 57 0.6530335 0.07158654
#> 58 0.6817608 0.07465357
#> 59 0.6164690 0.06767526
#> 60 0.6330833 0.06905155
#> 61 0.6983166 0.07951841
#> 62 0.5632053 0.06160674
#> 63 0.7274134 0.07943750
#> 64 0.5870984 0.06447442
#> 65 0.5575631 0.06086450
#> 66 0.6540060 0.07155874
#> 67 0.7275335 0.08365094
#> 68 0.7242167 0.07974692
#> 69 0.5727385 0.06297368
#> 70 0.7534651 0.08246257
#> 71 0.6206721 0.06781010
#> 72 0.7292623 0.08363080
#> 73 0.7282632 0.08000209
#> 74 0.7260446 0.08342532
#> 75 0.6509620 0.07135311
#> 76 0.7320781 0.08037407
#> 77 0.5004379 0.05470836
#> 78 0.7035650 0.07701909
#> 79 0.6497536 0.07452058
#> 80 0.6086334 0.06660682
#> 81 0.6351726 0.06979702
#> 82 0.5572917 0.06103033
#> 83 0.6455249 0.07063372
#> 84 0.5747477 0.06304723
#> 85 0.7766411 0.08473669
#> 86 0.6909958 0.07922860
#> 87 0.6501781 0.07136328
#> 88 0.7020855 0.07699626
#> 89 0.6724963 0.07383678
#> 90 0.6834803 0.07476896
#> 91 0.7307458 0.08021467
#> 92 0.6672748 0.07318789
#> 93 0.6437801 0.07050095
#> 94 0.7236141 0.07922690
#> 95 0.7028308 0.07701578
#> 96 0.5913975 0.06503644
#> 97 0.5044966 0.05519188
#> 98 0.7597918 0.08323918
#> 99 0.5666311 0.06472956
if (FALSE) { # \dontrun{
# Pairwise IA with p-values (this will take about a minute)
res <- pair.ia(partial_clone, sample = 999)
head(res)
# Plot the results of resampling rbarD.
library("ggplot2")
Pinf.resamp <- resample.ia(Pinf, reps = 999)
ggplot(Pinf.resamp[2], aes(x = rbarD)) +
geom_histogram() +
geom_vline(xintercept = ia(Pinf)[2]) +
geom_vline(xintercept = ia(clonecorrect(Pinf))[2], linetype = 2) +
xlab(expression(bar(r)[d]))
# Get the indices back and plot the distributions.
nansamp <- ia(nancycats, sample = 999, valuereturn = TRUE)
plot(nansamp, index = "Ia")
plot(nansamp, index = "rbarD")
# You can also adjust the parameters for how large to display the text
# so that it's easier to export it for publication/presentations.
library("ggplot2")
plot(nansamp, labsize = 5, linesize = 2) +
theme_bw() + # adding a theme
theme(text = element_text(size = rel(5))) + # changing text size
theme(plot.title = element_text(size = rel(4))) + # changing title size
ggtitle("Index of Association of nancycats") # adding a new title
# Get the index for each population.
lapply(seppop(nancycats), ia)
# With sampling
lapply(seppop(nancycats), ia, sample = 999)
# Plot pairwise ia for all populations in a grid with cowplot
# Set up the library and data
library("cowplot")
data(monpop)
splitStrata(monpop) <- ~Tree/Year/Symptom
setPop(monpop) <- ~Tree
# Need to set up a list in which to store the plots.
plotlist <- vector(mode = "list", length = nPop(monpop))
names(plotlist) <- popNames(monpop)
# Loop throgh the populations, calculate pairwise ia, plot, and then
# capture the plot in the list
for (i in popNames(monpop)){
x <- pair.ia(monpop[pop = i], limits = c(-0.15, 1)) # subset, calculate, and plot
plotlist[[i]] <- ggplot2::last_plot() # save the last plot
}
# Use the plot_grid function to plot.
plot_grid(plotlist = plotlist, labels = paste("Tree", popNames(monpop)))
} # }
# Resampling
data(Pinf)
resample.ia(Pinf, reps = 99)
#> Ia rbarD
#> 1 0.6326707 0.06954708
#> 2 0.6883222 0.07541424
#> 3 0.6740923 0.07387103
#> 4 0.5770239 0.06334574
#> 5 0.6091020 0.06637954
#> 6 0.5727045 0.06257368
#> 7 0.7155065 0.08212542
#> 8 0.6602011 0.07599176
#> 9 0.6471711 0.07096785
#> 10 0.5719934 0.06288824
#> 11 0.7141845 0.07861272
#> 12 0.6605182 0.07202927
#> 13 0.5484642 0.06268892
#> 14 0.7052128 0.07729392
#> 15 0.6276769 0.07197724
#> 16 0.6380409 0.06991244
#> 17 0.6086037 0.06975659
#> 18 0.6020064 0.06584930
#> 19 0.6331831 0.06928114
#> 20 0.6693489 0.07317111
#> 21 0.5524509 0.06033854
#> 22 0.6227598 0.07126893
#> 23 0.6450267 0.07040975
#> 24 0.6631087 0.07652916
#> 25 0.6463076 0.07083580
#> 26 0.6238613 0.06817980
#> 27 0.4787489 0.05220704
#> 28 0.7371110 0.08042796
#> 29 0.5515054 0.06044891
#> 30 0.6232699 0.06820601
#> 31 0.7071895 0.07748315
#> 32 0.6639126 0.07270494
#> 33 0.7081194 0.07737906
#> 34 0.6640895 0.07625090
#> 35 0.5629285 0.06154621
#> 36 0.5949440 0.06501098
#> 37 0.4891759 0.05349182
#> 38 0.6563514 0.07168735
#> 39 0.7800728 0.09000387
#> 40 0.6088116 0.06673665
#> 41 0.5569843 0.06091959
#> 42 0.6360185 0.06954124
#> 43 0.6308747 0.06906819
#> 44 0.7320468 0.08394502
#> 45 0.6802496 0.07450185
#> 46 0.5492261 0.06031984
#> 47 0.6919209 0.07928974
#> 48 0.6616311 0.07240787
#> 49 0.5632249 0.06183149
#> 50 0.6618410 0.07272939
#> 51 0.6293655 0.06914113
#> 52 0.7046288 0.07706778
#> 53 0.6398762 0.07020089
#> 54 0.7105198 0.07794080
#> 55 0.5990801 0.06551082
#> 56 0.7076892 0.07796176
#> 57 0.6530335 0.07158654
#> 58 0.6817608 0.07465357
#> 59 0.6164690 0.06767526
#> 60 0.6330833 0.06905155
#> 61 0.6983166 0.07951841
#> 62 0.5632053 0.06160674
#> 63 0.7274134 0.07943750
#> 64 0.5870984 0.06447442
#> 65 0.5575631 0.06086450
#> 66 0.6540060 0.07155874
#> 67 0.7275335 0.08365094
#> 68 0.7242167 0.07974692
#> 69 0.5727385 0.06297368
#> 70 0.7534651 0.08246257
#> 71 0.6206721 0.06781010
#> 72 0.7292623 0.08363080
#> 73 0.7282632 0.08000209
#> 74 0.7260446 0.08342532
#> 75 0.6509620 0.07135311
#> 76 0.7320781 0.08037407
#> 77 0.5004379 0.05470836
#> 78 0.7035650 0.07701909
#> 79 0.6497536 0.07452058
#> 80 0.6086334 0.06660682
#> 81 0.6351726 0.06979702
#> 82 0.5572917 0.06103033
#> 83 0.6455249 0.07063372
#> 84 0.5747477 0.06304723
#> 85 0.7766411 0.08473669
#> 86 0.6909958 0.07922860
#> 87 0.6501781 0.07136328
#> 88 0.7020855 0.07699626
#> 89 0.6724963 0.07383678
#> 90 0.6834803 0.07476896
#> 91 0.7307458 0.08021467
#> 92 0.6672748 0.07318789
#> 93 0.6437801 0.07050095
#> 94 0.7236141 0.07922690
#> 95 0.7028308 0.07701578
#> 96 0.5913975 0.06503644
#> 97 0.5044966 0.05519188
#> 98 0.7597918 0.08323918
#> 99 0.5666311 0.06472956
if (FALSE) { # \dontrun{
# Pairwise IA with p-values (this will take about a minute)
res <- pair.ia(partial_clone, sample = 999)
head(res)
# Plot the results of resampling rbarD.
library("ggplot2")
Pinf.resamp <- resample.ia(Pinf, reps = 999)
ggplot(Pinf.resamp[2], aes(x = rbarD)) +
geom_histogram() +
geom_vline(xintercept = ia(Pinf)[2]) +
geom_vline(xintercept = ia(clonecorrect(Pinf))[2], linetype = 2) +
xlab(expression(bar(r)[d]))
# Get the indices back and plot the distributions.
nansamp <- ia(nancycats, sample = 999, valuereturn = TRUE)
plot(nansamp, index = "Ia")
plot(nansamp, index = "rbarD")
# You can also adjust the parameters for how large to display the text
# so that it's easier to export it for publication/presentations.
library("ggplot2")
plot(nansamp, labsize = 5, linesize = 2) +
theme_bw() + # adding a theme
theme(text = element_text(size = rel(5))) + # changing text size
theme(plot.title = element_text(size = rel(4))) + # changing title size
ggtitle("Index of Association of nancycats") # adding a new title
# Get the index for each population.
lapply(seppop(nancycats), ia)
# With sampling
lapply(seppop(nancycats), ia, sample = 999)
# Plot pairwise ia for all populations in a grid with cowplot
# Set up the library and data
library("cowplot")
data(monpop)
splitStrata(monpop) <- ~Tree/Year/Symptom
setPop(monpop) <- ~Tree
# Need to set up a list in which to store the plots.
plotlist <- vector(mode = "list", length = nPop(monpop))
names(plotlist) <- popNames(monpop)
# Loop throgh the populations, calculate pairwise ia, plot, and then
# capture the plot in the list
for (i in popNames(monpop)){
x <- pair.ia(monpop[pop = i], limits = c(-0.15, 1)) # subset, calculate, and plot
plotlist[[i]] <- ggplot2::last_plot() # save the last plot
}
# Use the plot_grid function to plot.
plot_grid(plotlist = plotlist, labels = paste("Tree", popNames(monpop)))
} # }
